
Sputnik V is a vaccine that has saved many lives, and a cause for pride: Russia is one of the few countries capable of independent vaccine development. On the other hand, there have been so many irregularities, misinformation and incompetence in the process of developing, producing and distributing the vaccine that the drug has a mixed reputation. In fact, it reflects, like a drop of water, the problems of Russia's science, pharmaceutical industry, and PR. Inbio Ventures' Head of Scientific Research Ilya Yasny reviews the facts and speculation around Sputnik and ponders why Europe and the WHO have not yet approved the Russian vaccine.
Disclaimer: The author and his family were vaccinated with Sputnik.
First of all, it is necessary to distinguish three things:
- the principle of how Sputnik works, which is quite reasonable and similar to the Johnson & Johnson, CanSino and AstraZeneca vaccines;
- how the vaccine research was conducted;
- statements by the staff of the Gamaleya Center and the Russian Direct Investment Fund (RDIF) accompanying the development and subsequent stages of vaccine distribution.
Background: Ebola and a Criminal Case
The public history of Sputnik V began on April 20, 2020, when the director of the Gamaleya Institute, Alexander Gintsburg, said at a meeting with Putin that clinical trials of the vaccine could begin on June 1, and registration was possible from June 15. Of course, the government decree allows for a summary procedure for drug research during a pandemic, but two weeks for clinical trials is, to put it mildly, an optimistic forecast.
True, Gintsburg and other employees constantly refer to the fact that the platform on which Sputnik is made is well studied, and it's already the third vaccine obtained using the technology. Indeed, a vaccine for Ebola had been previously registered in Russia, but it had been done in violation of any rules, on the results of phase 1/2 alone, and phase 3 results have never been published anywhere; the research permit was added to the register retroactively.
There is one more “juicy” detail: in fact, Sputnik is not the third, but the fourth attempt of the Gamaleya Institute to create a vector vaccine: the first was AdeVac-Flu, which Gintsburg has not mentioned in recent times. Perhaps because the money for its creation was stolen, and a former employee of the Gamaleya Institute Rustam Ataullakhanov is accused of embezzling 424 million rubles from Rosnano. Gintsburg and his deputy Denis Logunov were lucky enough to be witnesses at the trial.
Hasty registration
In May 2020, a scandal erupted over testing of the vaccine on employees of the Gamaleya Institute even before testing it on animals. Director Gintsburg said it had nothing to do with clinical research. Although, according to every international rule, unregistered new drugs should be administered to people only as part of clinical trials: test subjects must sign an informed consent, take out insurance and should be carefully monitored, because their health and life are at great risk.
Finally, the registration of the vaccine on August 11 without any published data became a real sensation not only in Russia, but also abroad, and had an ambiguous effect on the reputation of the vaccine. All that was available for two months was scanty data from a poorly written manual, from which, however, it was clear that only twenty people received both doses of the vaccine in liquid form. This is not even close to a phase 2 study, let alone a registration study. For comparison, at that time the details on the first two phases of Western vaccine research, Pfizer/BioNTech, AstraZeneca, Moderna, had already been published in peer-reviewed scientific journals, and there were dozens of times more test subjects involved, but no one was in a hurry to register a vaccine using the preliminary data.
In addition, the statements accompanying the registration to the effect that it is the world's first registered vaccine against Coronavirus did not correspond to reality: on June 25, the Chinese vaccine CanSino was registered for use by the military. Equally ridiculous was the statement by the developer Logunov that temporary registration was necessary for getting the vaccine to risk groups. This contradicted both world practice and Russian guidelines for clinical trials.
Publications and trials
In September 2020, The Lancet published the results of phase 1/2 studies, which were heavily criticized by the Italian fighter against pseudoscience Enrico Bucci and other scientists who made allegations of data falsification. Logunov's team responded to the criticism, but the primary data, by which it would have been possible to judge with confidence whether or not there was a falsification, were never provided. Besides the criticism, the paper had many shortcomings. There was some ethical concern over the fact that members of the military were involved in the study; it is a dependent group, and special care is needed to ensure that participants are volunteers and well-informed.
In September, a phase 3 trial began. Unlike those of all leading vaccine manufacturers, Sputnik's protocol has not been published. Meanwhile, the protocol is the key document of clinical trials, describing the experimental design and indicating in advance what result (and based on what criterion) is considered successful by the researchers, how many volunteers are needed for that purpose, and other details. I was able to familiarize myself with the rough draft of the protocol written in August. At first glance, it is well written and similar to the protocols of Western companies, but there are some important nuances: the description of whether a person is sick with Covid or not is not detailed enough and rather subjective, which largely leaves the decision for the doctor. This allows for the data to be manipulated if it's known who is in the placebo group and who got the vaccine. Given the fact that the vaccine causes adverse reactions more often, such an approach may lead to an overstatement of its efficacy.
The description of whether a person is sick with Covid or not is rather subjective, which largely leaves the decision for the doctor
Unlike the protocols of other companies, the Sputnik protocol has no intermediate test points. In the meantime, waiting 180 days after the inclusion of the last patient in the study is indeed too long, so from the publication about phase 3 we learn that the interim tests were included in the protocol in November. Changing the phase 3 protocol when the study is already underway can invalidate the results, and great care is required along with sufficient substantiation to the effect that everything was done correctly. As you might guess, the paper had no mention of that.
The situation with the efficacy of «Sputnik» against new strains is also unclear. The Gamaleya Institute, of course, claims that it is effective, without giving any evidence (wait, they say, for a publication in May). Independent researchers analyzed serum taken from vaccinated Argentinians and found it was less effective in neutralizing the South African strain of the virus.
Production
Simultaneously with phase 3, the vaccine production began scaling up and Generium, Biocad, R-Pharm and other companies became involved in the process. The transfer of production to commercial companies raised hopes that the vaccine would be of high quality, but at the same time raised the question of the comparability of products produced at different sites. For example, a product produced at the Gamaleya Institute in 5-liter reactors for clinical research does not have to be the same as that produced by Generium in a 1000-liter reactor for mass use. Their comparability requires separate studies. Incomplete comparability is implied by differing storage and use instructions contained in manuals for the drug made by different manufacturers.
From thereon onwards, the promises for the number of doses constantly surpassed the actual rates of production. However, the fact that production could not be scaled up right away is not surprising - Russia had no experience of independent production and scaling-up of high-quality innovative drugs, and did not start purchasing scaling-up equipment until the end of September. In total, as of May 12, Russia had produced 33 million doses, of which 15 million were exported. Thus, Mexico received 1.9 million doses out of the 24 million promised under the contract. The Mexican Ministry of Health said it was due to problems with the production of the second dose, but RDIF claims this is incorrect.
Finally, at the end of November, preliminary results of the third phase were announced for all leading vaccines, including Sputnik. Like Pfizer and Moderna, Sputnik's efficacy was 95%. The data were published in the form of press releases, but for Western vaccines, more detailed information became quickly available after the approval of the vaccines in the US and the EU – unlike the Ministry of Health of the Russian Federation, health agencies in those territories publish detailed information from both the manufacturer and the regulator; a video of the scientific advisory committee meeting is also available in the US. Details about Sputnik had to wait until February 2021, when a paper was published in the Lancet magazine about the results of phase 3. For the first time, the results of a study of the third phase of a Russian drug were published in such a prestigious international journal. Although, in regard to that publication researchers still had some questions that could have been largely resolved if the research protocol and primary data had been provided. In particular, it turned out that the reactogenicity of the vaccine was not studied at all. According to the manual, after vaccination, information on how patients react to the vaccine should be collected over several days by means of interviewing the patients. The protocol did not provide for it.
Comparability of vaccines produced at different sites needs to be investigated separately
Yet, in November-December 2020, information from Telegram chats about those vaccinated with Sputnik started piling up. Both the elderly and people with concomitant diseases did not experience any serious side effects and produced antibodies. This ultimately convinced me to advise my parents to get vaccinated. Of course, such a situation is abnormal; official data rather than social networks should be the source of reliable information about medicines.
True, we now have data on actual application of the vaccine from Argentina and San Marino (but not from Russia). Only the safety of the vaccine can be reliably assessed - the number of side effects in the San Marino study is at the upper threshold for the other vaccines. Researcher Hilda Bastian, one of the founders of the Cochrane Society and an expert in evidence-based medicine, has been conducting a comparison of the efficacy and safety of vaccines with a reservation to the effect that hers is an indicative chart, because it is not entirely correct to directly compare data from different studies. The data for mid-May are shown in the picture below.
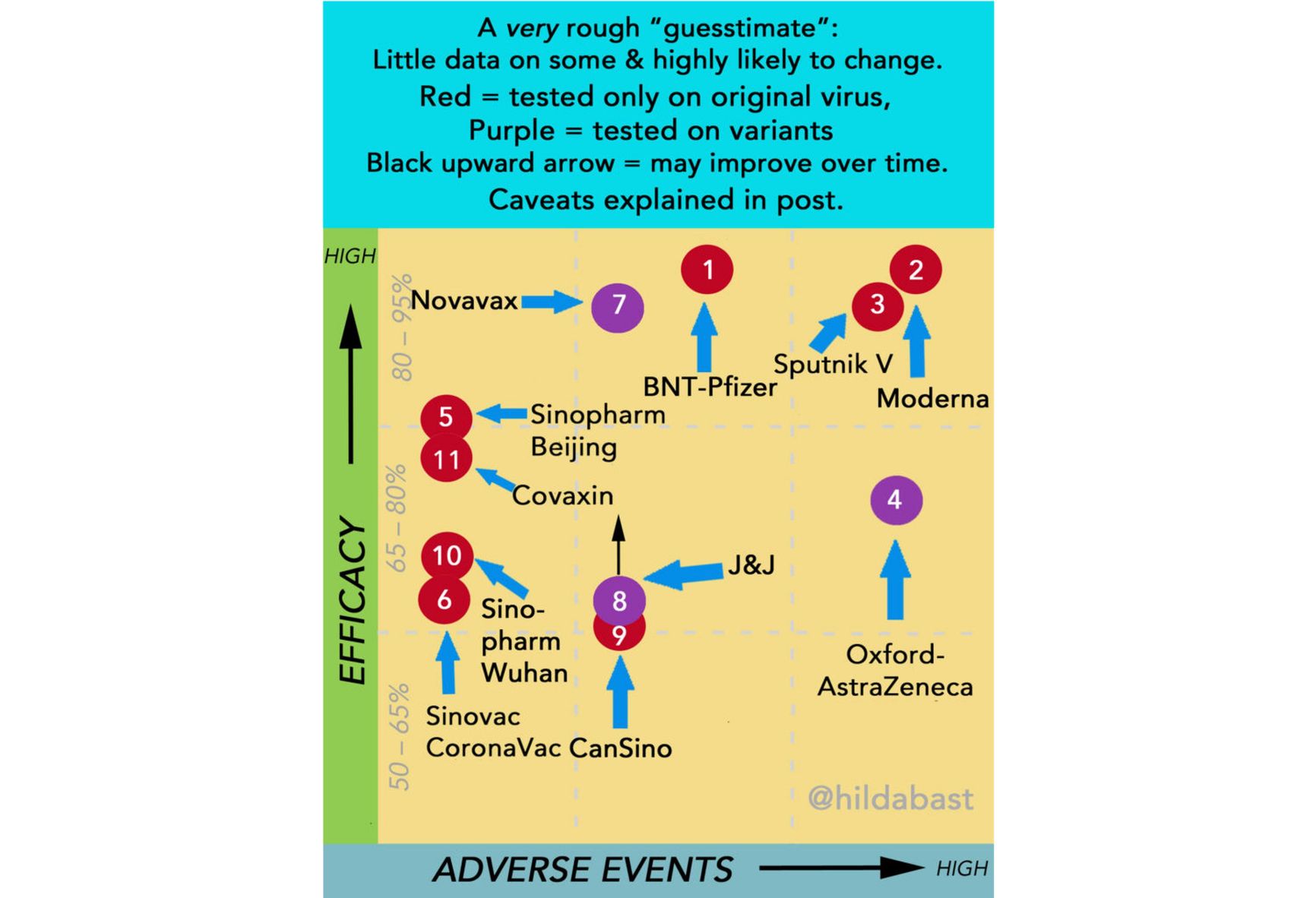
As we can see, Sputnik has a high declared efficacy, but also a high level of side effects. The number of new Covid cases in San Marino dropped to zero, given that 80% of the population got the Sputnik vaccine.
Export abroad
Other countries have been showing their interest in Sputnik: by November 2020, applications were received from 50 countries for a total volume of 1.2 billion doses. However, already in December, the first conflicts emerged - Turkey refused to buy Sputnik V due to the inconsistency of the preclinical studies with good laboratory practice (GLP). These are the standards that ensure the quality of animal studies, primarily for toxicity. If they are not followed, one cannot be sure that the drug has been well tested for safety.
In response, Ginzburg said that “laboratory production is certified according to the standards of the Ministry of Industry and Trade, but not according to those of the European certification agencies. We do not have a European certificate, but we have a Russian one, like our entire industry.» It is a very strange answer - Turkey's claim was not about production at all, but about preclinical research. Laboratory production is not subject to certification, only industrial production is supposed to be licensed. The licensing standards of the Ministry of Industry and Trade are almost a copy of the European standards.
Turkey said it would conduct its own research on the vaccine. Having done so, the Turks approved the vaccine on April 30 and purchased 50 million doses. They have also been organizing their own production.
In general, for context, it is important to understand that the whole world is divided into two large zones – the countries that adopted the modern standards for drugs developed by ICH (The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use) and the other countries. The USA, the EU, Japan, Switzerland, and Canada have the strictest and the most up-to-date regulations. It is important to emphasize that those guidelines are not bureaucratic obstacles in the way of manufacturers, erected by the evil “Big Pharma”, but a harmonious, scientifically grounded system that finally emerged after many incidents and that provides the population of these countries with safe, effective and high-quality medicines.
Two EU countries, Hungary and Slovakia, have entered into negotiations with RDIF on the supply of Sputnik despite the lack of approval from the EMA and the WHO. Hungary approved the vaccine back in January 2021, but continued its internal checks. Mass vaccination of the population began in February. With Slovakia, it turned out to be more difficult: the approval of the vaccine and the decision to purchase Sputnik led to a domestic political scandal. Then, having received the first vaccine samples, the Slovaks said they did not correspond to what was published in the Lancet, and the data from the dossier was not sufficient to pass judgement on the safety and efficacy of the vaccine.
In response, RDIF demanded the return of the delivered 200,000 doses, citing a violation of the contract by the Slovaks, according to which the vaccine could only be tested in EU-certified laboratories. Then Slovakia published a contract, from which it is clear it has no such provisions. However, on May 9, Slovakia reported that the doses sent to Hungary for testing were of satisfactory quality, and approved the vaccine for domestic use on May 26. Earlier, the Czech regulator claimed the data in the dossier were insufficient and they would stop the consideration of the documents.
Probably the loudest scandal erupted in Brazil. The local regulatory agency ANVISA announced it would not allow the registration of Sputnik V for the time being due to insufficient data and several issues that concerned the agency:
- the product documentation sets a fairly high bar for the detection of replicating adenovirus. If such an adenovirus can actually be detected, it does not correspond to the statements of the Russian side about its absence, and in this case, stricter requirements for its safety studies should be imposed. In particular, such adenovirus can accumulate in tissues. The matter attracted the most public attention, and in response the Gamaleya Institute said they simply had been in a hurry and hadn't had enough time to validate the more rigorous tests. In fact, the levels of replicating adenovirus are within the acceptable limits;
- there is no information on controlling the presence of other viruses in the drug, and the information on controlling the production process for the presence of impurities is not complete;
- the phase 3 clinical trial protocol does not clearly describe the disease criteria, the algorithm for collecting data on adverse phenomena, and some other aspects of the study, which increases the possibility of data manipulation;
- no reactogenicity data were collected;
- there is no data on comparability between series of 5-liter batches produced for clinical trials at the Gamaleya Institute and series of commercial batches, hundreds of liters in volume;
- an audit conducted by the Brazilians at the Generium and UfaVita sites revealed shortcomings in production processes, particularly the risk of violation of sterility standards;
- the Brazilians were not allowed to enter the Gamaleya site at all.
RDIF has promised to sue ANVISA for libel. But some of those comments were predictable even before ANVISA, and in any case, they will have to be addressed before the EMA or the WHO approves the vaccine. If RDIF and Gamaleya want to register the vaccine in the European Union (and they were given such an order, as they say, «at the very top»), they will have to let EMA auditors visit even such a top-secret facility as the Gamaleya Institute.
Meanwhile, in Brazil, the local company União Química set up its own production of Sputnik under license and applied for registration. The Indian Dr. Reddy's followed suit, and the Indians also conducted their own clinical research; they have been setting up the world's largest Sputnik V production.
Black and White PR
It's time to mention the stance RDIF's PR people take towards every attempt to criticize the vaccine or to simply compare it to other analogues. From the very beginning, the fund chose a defensive-aggressive strategy, rather than openness and readiness for dialogue, with an emphasis on belittling the merits of other vaccines and proclaiming Sputnik's 97% efficacy. Thus, it wrote in a press release that the incident rate among 3.8 million vaccinated Russians was 0.027% in January-March 2021. This means that out of those vaccinated twice with Sputnik V, 1,026 people fell ill. At the same time, according to the Mosgorzdrav (the Moscow urban Health Authority) information, almost as many as 1,000 people out of a million vaccinated Muscovites fell ill by March 31. The contradiction in numbers is obvious.
RDIF representatives dismissed all signals from different countries mentioned above as «fake news», explaining them away as an «information attack» on Sputnik by the US State Department and the organizations under its control. True, the US state propaganda provided a pretext for it: according to the 2020 US Department of Health report, they «urged Brazil to abandon the Russian vaccine.» The report was bashfully removed from the State Department's website, but the cache remembers everything. As for the vilification of competitors, the story of the Hungarian table is the most egregious. RDIF published on Twitter a table showing infections and deaths caused by various vaccines, from which it can be deduced that the Pfizer/BioNTech vaccine has a mortality rate which is almost 20 times higher than Sputnik's.
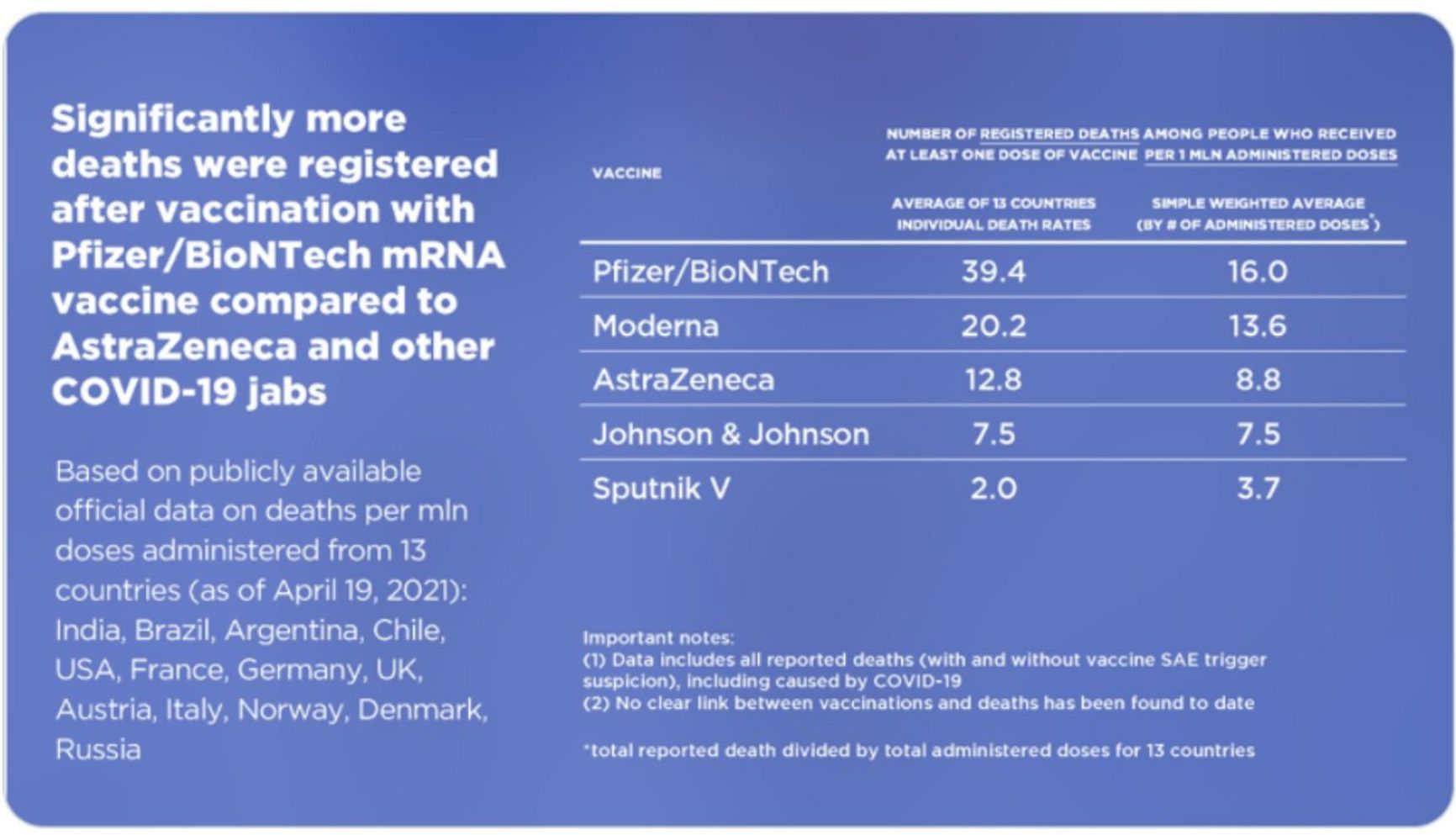
Comparison table from Sputnik V's Twitter
There are several problems with this table:
- the first column contains figures that are unscaled to the number of those vaccinated;
deaths from all causes are accounted for, not just from vaccinations (yes, road accidents and falling from heights are also included).
- age, incidence of Covid (and other diseases), quality of data collection – all play a key role in mortality data. No adjustment for any of this was introduced.
- the fact that the vaccines have been used for different periods of time has not been taken into account. Thus, in the US, the mortality rate is 10.4 per million for Pfizer, and 7.5 per million for J&J. But Pfizer has been used since December, and J&J since February. Of course, those who got Pfizer had more time to die!
- It should also be borne in mind that Pfizer was initially administered to the most elderly and vulnerable: it is clear (to everyone, except the victims of RDIF propaganda) that the mortality rate is much higher among them. On the contrary, chronically ill patients did not get Sputnik in Hungary.
“Black” PR of this kind, as we have said, is bad for all vaccines, including Sputnik.
What stands in the way of registration in the European Union?
If RDIF aims to register Sputnik in the EU, and the EU is still experiencing a shortage of vaccines, what is stopping the registration of the Russian drug right now? Many believe that the EMA is deliberately delaying the approval process for political reasons in order to annoy Russia. I am not a supporter of this point of view. The problems voiced by the Brazilian agency ANVISA do exist, and they are significant enough to prevent EMA registration. The agency must ascertain that the research conducted and the doses produced do indeed provide a quality drug which is as effective and safe as declared by the manufacturer.
Unfortunately, there was a scandal with EMA as well. RDIF had claimed since January that it had applied for vaccine registration in the EU. At the same time, there was no information about this on the EMA website, in contrast to the applications from other manufacturers. But RDIF stubbornly continued to claim the application was pending and the vaccine could be approved in early March. The EMA even had to issue a special press release stating that this was not true. In response, RDIF, of course, accused the EMA of spreading «fake news» (not the best way to build a dialogue with the most professional agency on the planet). Later, however, it turned out they had uploaded documents to the wrong website.
However, EMA was not offended and accepted the application on March 4. True, it was not an application for approval per se, but rather for a so-called rolling review, i.e., a gradual reviewing of the documents as they are submitted. When the agency concludes that the documents are sufficient to apply for registration and the benefits outweigh the risks, it will decide on registration.
The EMA and the WHO have joined forces to review the Sputnik application and have already conducted audits, the results of which remain unknown. A WHO document dated May 18 says that not all data from Gamaleya have been received yet, and audits will continue until June. This means the registration of Sputnik is unlikely to occur earlier than July. It is questionable whether by that time the vaccine will be needed in the EU in the quantities in which it is now being produced. However, if the drug is prequalified by the WHO, it will soon find its way to many developing countries under the COVAX program.
Conclusion
The situation with Sputnik is complicated. On the one hand, it is unique; on the other, it is quite expected. For the first time, a medicine from Russia came under the scrutiny of the top professionals around the globe. And, perhaps, deservedly so: the vaccine really saves lives and does not seem to have serious side effects. Sputnik has already been approved in more than 60 countries.
However, its birthmarks - non-transparency of development, restricted information, problems with PR, and even outright lies - greatly hinder Sputnik's worldwide promotion. We really hope these mistakes will be taken care of and corrected over time; after all, prior to being corrected they need to be acknowledged.
This article is also available in Russian